CANBIO2 – Projects
Individual projects
Bitte beachten Sie, dass der Inhalt nur auf Englisch verfügbar ist.
WP3 – Novel Treatment Strategies
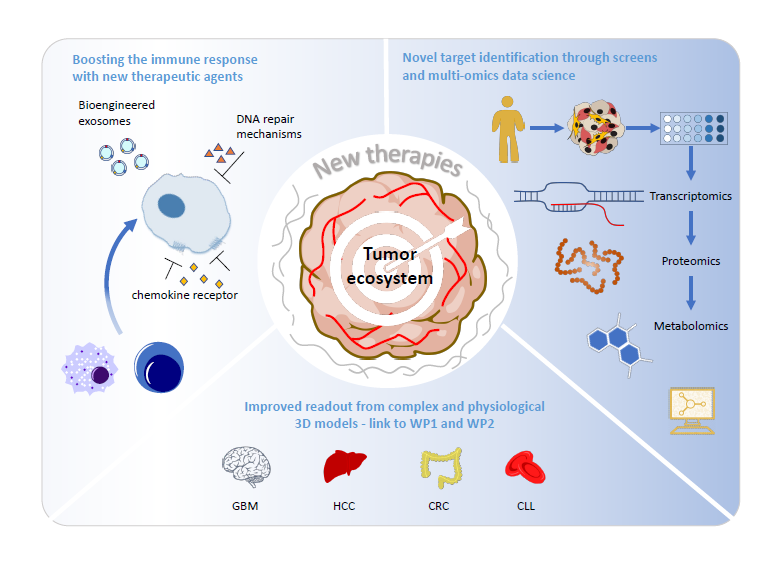
Project 13: Supervisor Prof Iris Behrmann (UL, Department of Life Sciences and Medicine, Esch-sur-Alzette): Finding new treatments for drug resistant hepatocellular carcinoma in physiologically relevant 3D in vitro models.
Recruitment closed. Position has been filled.
Drug resistance in hepatocellular carcinoma (HCC [35, 36]) has been shown to be associated with an altered cellular metabolism. Based on novel multi-component 3D cultures including Kupffer, stellate and endothelial cells, grown in physiologically relevant conditions a metabolic drug screen on drug-resistant and drug-sensitive HCC cells will be integrated with transcriptomic data for target identification.

Prof Iris Behrmann is Professor for Biochemistry and Head of the UL Department of Life Sciences and Medicine. Biologist by training, she did her PhD and post-doctoral research at the German Cancer Research Centre in Heidelberg before becoming group leader at the RWTH Aachen Medical School. In Luxembourg, her team in the Signal Transduction Laboratory works in the field of cancer and inflammation, in particular regarding cellular communication, drug resistance, and the modulatory effects of microenvironmental factors. Prof Behrmann’s research has contributed to the elucidation of the molecular signal transduction mechanisms of interleukin-6-type cytokines via the Jak/STAT pathway.
Project 14: Supervisor Dr Eric Van Dyck (LIH, Department of Cancer Research, Luxembourg): Harnessing DNA repair factors to promote an innate immune response against glioblastoma.
Recruitment closed. Position has been filled.
Preliminary evidence from the consortium suggests that the DNA repair status of GBM cells affects the recruitment of tumour-associated macrophages (TAMs) and the mounting of an innate immune response. Here we will investigate whether DNA repair-based strategies can contribute to turn GBM into an “immunologically hot” tumour. An RNAi screen will be performed in GBM cells to identify the DNA repair factors whose depletion promotes phagocytosis by macrophages.

Dr Eric Van Dyck is a molecular biologist and biochemist who heads the DNA Repair and Chemoresistance research group at LIH’s Department of Cancer Research. His group is developing approaches to better understand the DNA repair and epigenetic mechanisms that operate in adult and paediatric glioblastoma (GBM) and identify critical targets for innovative DNA repair-based therapeutic strategies. Recent achievements include the identification of a DNA repair and cell cycle gene expression signature in adult GBM with perspectives for precision medicine. Current research lines focus on the targeting of tumour propagating cells in GBM as well as on the interrelationship between DNA repair factors and the immune response in cancer.
Project 15: Supervisor Dr Petr Nazarov (LIH, Department of Cancer Research, Luxembourg): Deconvolution of complex molecular signals of metabolomics and transcriptomics for predicting drug sensitivity and therapy resistance in malignant melanoma.
Recruitment closed. Position has been filled.
Tumour and TE heterogeneity are reflected in multimodal data including e.g. genomic, transcriptomic and metabolic levels. However, data integration and sharing functional annotations between different data modalities, is still a challenging task. Recently we proposed [38, 39] a deconvolution-based method which allows to connect several modalities. This will be applied on transcriptomic and metabolic datasets from the consortium and public datasets for predicting drug sensitivity and associated biomarkers in melanoma.

Dr Petr Nazarov is leading the Multiomics Data Science research group and the Bioinformatics platform. His background is in data analysis, statistics, and machine learning methods, mainly in application to studying various bio-molecular systems. Both the research group and the platform are oriented at building advanced approaches to data analysis in (epi-)genomics, transcriptomics, proteomics, and metabolomics in cancer research. The specific focus of Dr Nazarov past years was on the deconvolution of molecular data in order to extract meaningful signals from cancer and stromal cells. He showed that such signals improve patient classification and can be used as prognostic markers.
Project 16: Supervisor Dr Johannes Meiser – DTU coordinator (LIH, Department of Cancer Research, Luxembourg): Integrative multi-omics analysis of IDH mutant gliomas to reveal novel targets for treatment.
Recruitment closed. Position has been filled.
Mutations in isocitrate dehydrogenase 1 or 2 (IDH1/2) define glioma subtypes and are primary events in gliomagenesis, impacting tumour epigenetics and metabolism. We have recently identified a novel metabolic vulnerability in the glutathione synthesis pathway that may be therapeutically exploited. Here we expand these studies leveraging integrated multi-omics data of patient samples including genetic/epigenetic, transcriptomic, metabolomic and proteomic data to reveal metabolic deficiencies in IDH wild-type and IDH mutant gliomas. Available drugs will be tested in patient-based tumour organoids and glioma xenografts available in the team.

Dr Johannes Meiser is leading the Cancer Metabolism Group at LIH’s Department of Cancer Research. During a successful postdoctoral stay at the Cancer Research UK Beatson Institute, he specialised in the field of cancer metabolism. In 2018, he successfully transitioned to a Principal Investigator position at LIH, core funded by the FNR ATTRACT program. The expertise of the Meiser lab resides at the quantitative analysis of mammalian cell metabolism applying stable isotope-assisted metabolic flux analysis and analytical chemistry using Mass Spectrometry. A particular focus of the group is set but not limited to folate-dependent serine metabolism.
Project 17: Supervisor Dr Anna Golebiewska (LIH, Department of Cancer Research, Luxembourg): Tumour plasticity as a treatment resistance mechanism in glioblastoma.
Recruitment closed. Position has been filled.
GBM cells display strong intrinsic plasticity and adapt reversibly to changing microenvironmental stimuli, forming a dynamic ecosystem. The role of tumour plasticity in creating treatment-resistant states is currently less understood. Here we will leverage existing and novel single cell RNA-Seq datasets to investigate transcriptomic changes in treatment-naive and treated tumours. We will apply advanced computational algorithms, including reference-free deconvolution methods, to reveal treatment-resistant states, their molecular signatures and regulators.

Dr Anna Golebiewska is Group leader of the NORLUX Neuro-Oncology laboratory. She has a background in molecular and cellular biology and obtained her PhD in stem cell research. Her work focuses on understanding brain tumour biology, in particular tumour heterogeneity and microenvironment. Her current projects aim to tackle intrinsic plasticity allowing brain tumour cells to adapt and survive external pressures from microenvironment and treatment. Her lab developed a large collection of glioma patient-derived organoids and orthotopic xenografts for preclinical research and drug testing, for which she received the FNR Award for Outstanding Scientific Achievement in 2021.
Project 18: Supervisor Dr Jérôme Paggetti (LIH, Department of Cancer Research, Luxembourg): Bioengineered small extracellular vesicles (sEV) as new cancer immunotherapies to counteract immune suppression.
Recruitment closed. Position has been filled.
sEVs represent a complex cell communication system that contributes to immune suppression in cancer through inhibitory immune checkpoint (IC) ligands at their surface. Here we will produce bioengineered sEVs displaying IC receptors acting as scavenger to counteract tumour-derived sEVs within the TME. Engineered sEVs with different combinations of IC receptors will be produced from stromal cells. Functionalities and efficacy of these sEVs will be investigated in advanced preclinical models using CyTOF, imaging flow cytometry and high-resolution microscopy.

Dr Jérôme Paggetti heads the Tumour Stroma Interactions group in LIH’s Department of Cancer Research since 2017. His research in haemato-oncology focuses on the interplay between leukemic cells and their microenvironment in particular the immune system, using patient samples and murine pre-clinical models. The group is composed of 14 members (5 PhD students, 5 postdocs, 1 engineer, 1 scientist and 2 group leaders). He established collaborations with well-known universities and cancer institutes in Europe, published in top journal of the field and received several scientific prizes including the FNR Award for Outstanding Publication in 2016.
Project 19: Supervisor Dr Andy Chevigné(LIH, Department of Infection & Immunity, Esch-sur-Alzette): Targeting the atypical chemokine receptor ACKR3 for improved cancer therapy.
Recruitment closed. Position has been filled.
The atypical chemokine receptor ACKR3/CXCR7 acts as a key regulator of TME-derived chemokines and controls cancer cell proliferation, survival and metastasis. Our team has recently developed and patented several ACKR3 modulators and identified three compounds blocking its capacity to scavenge chemokines. Here we will establish the anti-cancer activity of these drugs in different in vitro and in vivo models of increasing complexity (organoids/syngeneic/PDXs) available within the consortium, including glioblastoma, breast cancer and leukaemia/lymphoma.

Dr Andy Chevigné studied Biochemistry at the University of Liège (Belgium) and obtained his PhD from the Centre for Protein Engineering (ULiège) and the Laboratory of Applied Genetics (Free University Brussels, ULB). He joined LIH in 2008 to investigate the role of chemokine receptors in HIV-1 entry and became independent group leader in 2011. He is currently Head of the Immuno-Pharmacology and Interactomics group, which has as main research focus the study of chemokine receptors. He is also Deputy Head of Academic affairs at LIH and Lecturer at ULiège. He was co-awarded the Galien prize of Pharmacology 2019. He supervised several postdocs and PhD students with support from funding agencies and private companies.
Project 20: Supervisor Ángel Álvarez-Prado (LIH, Department of Cancer Research, Strassen): Decoding the impact of genetic variation on anti-tumoral immunity and immunotherapy efficacy in brain metastatic tumors.
Brain metastases (BrMs) are the most common brain tumors in adults, and are linked to a dismal prognosis. Therefore, there is an urgent need for more effective treatments. Previous research linked the genetic makeup of cancer cells to the abundance and function of immune cells in the microenvironment of human BrMs, suggesting an important role for somatic variation in shaping the TIME. Our hypothesis is that genetic variation instructs specific immunophenotypes in the microenvironment of BrM tumors, which in turn shape responses to therapy. Therefore, this project aims to: (i) evaluate the efficacy of radio- and ICB therapy in preclinical mouse models of lung- and melanoma-BrM bearing different genetic makeups; (ii) characterize the TIME in treated and untreated tumors from these models; (iii) explore the link between somatic genetic variation, TIME phenotypes and responses to therapy in human BrMs.

The Translational Cancer Immunogenomics (TCI) Research Group, led by Dr. Ángel Álvarez-Prado at the Department of Cancer Research of LIH, is currently offering a Ph.D. student position. Recently established, the research group focuses on understanding how genetic alterations in cancer cells shape the immune microenvironment of brain metastatic tumors, with the long-term goal of developing novel personalized immunotherapies for cancer patients with brain metastasis.
Project 21: Supervisor Dr. Sabrina Fritah (LIH, Department of Cancer Research, Strassen): Integrative multi-omics analysis of IDH mutant gliomas to reveal novel targets for treatment.
Mutations in isocitrate dehydrogenase 1 or 2 (IDH1/2) define glioma subtypes and are primary events in gliomagenesis, impacting tumor epigenetics and metabolism. We have recently identified a novel metabolic vulnerability in the glutathione synthesis pathway that may be therapeutically exploited. Here we expand these studies leveraging integrated multi-omics data of patient samples including genetic/epigenetic, transcriptomic, metabolomic and proteomic data to reveal other metabolic deficiencies in IDH wildtype and IDH mutant gliomas. Our preliminary data indicate that rewiring of the butyrate pathway could reveal a novel metabo-epigenetic regulation, associated with an alteration in the gut-brain axis crosstalk in glioma. We will apply epigenetic drugs on CRISPR-engineered glioma models to test their impact on metabolic and epigenetic regulations and glioma features.

Recently established, the Cancer RNAs and Epigenetic Group (CREG) laboratory focuses on understanding the role of epigenetic and epitranscriptomic regulation in tumor progression, with the ultimate aim to develop innovative nucleic acid therapeutics against cancer. We make use of advanced molecular and cellular technologies to identify novel targets and get mechanistic insights into disease pathways.

discover CANBIO2
Projects – WP1 – Novel Cancer Models
Projects – WP2 – Cancer Metabolism
Projects – WP3 – Novel Treatment Strategies
Contact
For any question related to CANBIO2, please contact: