CANBIO2 – Projects
Individual projects
Bitte beachten Sie, dass der Inhalt nur auf Englisch verfügbar ist.
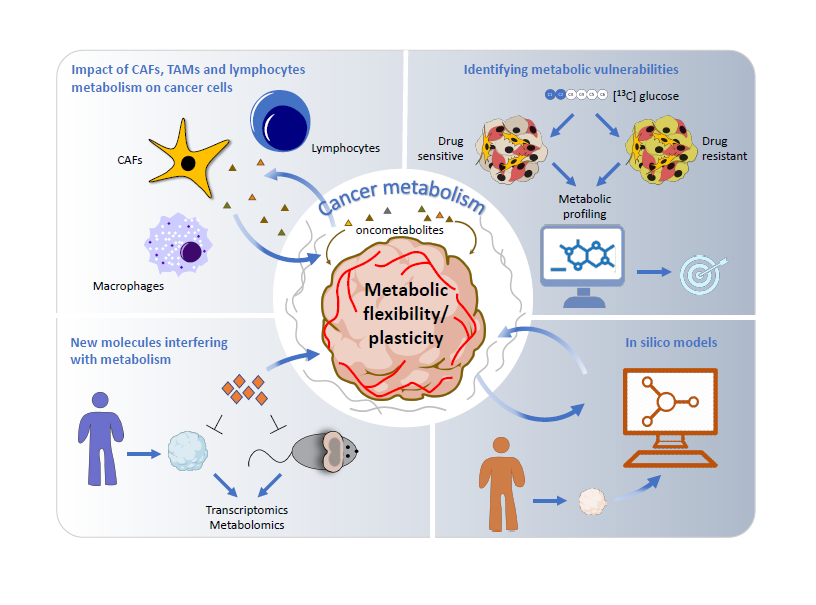
WP2 – Cancer Metabolism
Project 8: Supervisor Dr Johannes Meiser – DTU co-coordinator (LIH, Department of Cancer Research, Luxembourg): Identifying metabolic vulnerabilities in 3D.
Recruitment closed. Position has been filled.
A prerequisite for metabolic studies in advanced cancer models, is the adaptation of respective protocols to account for the experimental and analytical challenges that go along with the transition from 2D to complex 3D models. Here, we will translate our robust profiling pipeline to advanced 3D cancer models. Taking advantage of different 3D models available within the consortium, the profiling pipeline will be first benchmarked by validating known metabolic alterations in melanoma, CRC and glioma. Subsequently, metabolic profiling upon TE-relevant constraints (hypoxia and nutrient deprivation) will be applied. This approach may also help to identify novel disease-relevant entry points to counteract drug resistance (P1, 13, 16). In the long run, this pipeline will also be used to create tumour type-specific metabolic maps with the aim to make it available to the cancer research community.

Dr Johannes Meiser is leading the Cancer Metabolism Group at LIH’s Department of Cancer Research. During a successful postdoctoral stay at the Cancer Research UK Beatson Institute, he specialised in the field of cancer metabolism. In 2018, he successfully transitioned to a Principal Investigator position at LIH, core funded by the FNR ATTRACT program. The expertise of the Meiser lab resides at the quantitative analysis of mammalian cell metabolism applying stable isotope-assisted metabolic flux analysis and analytical chemistry using Mass Spectrometry. A particular focus of the group is set but not limited to folate-dependent serine metabolism.
Project 9: Supervisor Dr Etienne Moussay (LIH, Department of Cancer Research, Luxembourg): Overcoming treatment resistance with new therapeutic opportunities in leukaemia.
Recruitment closed. Position has been filled.
Clinical treatment options in chronic lymphocytic carcinoma (CLL) are often limited due to fast occurring drug resistances. The specific TE of such tumours are lymph nodes, which harbour the proliferating pool of cancer cells. Here, different novel small molecules will be investigated in regard to their potential to overcome treatment resistance of CLL cells. One of them being a novel small molecule targeting the one-carbon metabolism, a pathway that is essential to support nucleotide synthesis. The project will take advantage of patient-derived cells, genetic mouse models and resistant cell lines.

Dr Etienne Moussay studied Cellular Biology and Physiology before obtaining his PhD in Immunology and Cell Biology. He is co-heading the Tumour Stroma Interactions group and focuses his research on hematologic malignancies, and chronic lymphocytic leukaemia in particular. With patient samples and transgenic murine models, he studies tumour immunology, the benefits of immunotherapy and the communication between cells in the tumour microenvironment (TME). He aims at finding vulnerabilities and testing innovative therapies to stop the supportive effect of the TME and eradicate the disease. He is the recipient of two FNR Awards for his work on microRNA and extracellular vesicles in leukemia.
Project 10: Supervisor Prof Dirk Brenner (LIH, Department of Infection & Immunity, Esch-sur-Alzette): Anti-Tumour Immunity: Tuning metabolic circuits in T-cells to fight cancer.
Recruitment closed. Position has been filled.
Since the advent of immune-checkpoint blockade, T-cells gained much attention in regard to their anti-cancer properties. It has also become obvious that alterations of the metabolic landscape impact T-cell functionality. Yet, an in-depth understanding of metabolic determinants that control phenotypes is only starting to arise. A recent metabolic screen (unpublished) performed in the Brenner lab has uncovered new regulators that impact the immune response of cytotoxic T-cells. These identified targets will be mechanistically characterised to understand their role in the immunological circuit. In addition to ex vivo T-cell assays, genetically engineered mouse models will be employed.

Prof Dirk Brenner is Deputy Director (Research & Strategy) of the LIH’s Department of Infection and Immunity and Full Professor of Immunology & Genetics at the Luxembourg Centre for Systems Biomedicine of the University of Luxembourg. In addition, he is an Adjunct Professor of Allergology at the University of Southern Denmark and serves as co-coordinator of the study group ‘Signal Transduction’ within the German Society of Immunology (DGfI). His major interests lie in unraveling metabolic and molecular regulatory circuits within the adaptive and the innate immune system. By integrating in vitro with in vivo studies his group aims to gain a comprehensive picture of inflammation and cancer.
Project 11: Supervisor Dr Alessandro Michelucci (LIH, Department of Cancer Research, Luxembourg): Elucidating the role of the IRG1/ACOD1-itaconate axis in TAMs polarization in glioblastoma.
Recruitment closed. Position has been filled.
While GBMs are characterised by very little lymphocytic infiltration, myeloid cells (microglia in particular) can represent up to 20 % of the tumour mass. In this project, the role of the immuno-metabolite itaconic acid will be studied by employing an IRG1 knock-out mouse model. Preliminary data of the Michelucci lab have highlighted that loss of IRG1 results in an increased number of lymphocyte infiltration, indicating an anti-tumorigenic phenotype upon loss of IRG1 in myeloid cells. The mechanistic details underpinning this phenotype will be studied.
The project foresees the combination of wet lab and bioinformatics analyses, the latter being a pre-requisite to be developed with the support of our bioinformatics core facility.

Dr Alessandro Michelucci is a cellular and molecular biologist leading the Neuro-Immunology group focused on myeloid cells and neuroinflammation in brain tumours and neurodegenerative diseases. His current research aims at studying the molecular mechanisms underlying the glioma microenvironment. Specifically, he investigates the contribution of microglia/macrophages in tumour escape mechanisms by analysing their phenotypic and functional adaptation in the tumorigenic process in order to reprogram them to promote anti-tumour activities. In 2014, he received the FNR Award for Outstanding Scientific Publication for the discovery of the function of a key gene implicated in the emerging field of immunometabolism.
Project 12: Supervisor Prof Thomas Sauter (UL, Department of Life Sciences and Medicine, Esch-sur-Alzette): Unravelling metabolic effects in the microenvironment of head and neck squamous cell carcinoma applying a systems biology approach.
Recruitment closed. Position has been filled.
In addition to immune cells, stromal cells are another well-known determinant of cancer biology, for example by fibroblast-derived collagen excretion or stellate cell-derived alanine. To better understand the complex interplay of CAFs in context of head and neck squamous cell carcinoma (HNSCC) computational approaches will be leveraged (e.g. pathway enrichment methods and genome scale metabolic network modelling) to enable an un-biased large-scale metabolome analysis of clinical samples. The in silico approach will be accompanied by CAF-cancer cell co-culture models and will be fuelled by an established collaboration to King’s College London.

Prof Thomas Sauter, a computational biologist and engineer is leading the Systems Biology group focused on Model based Data Integration and Analysis of Disease specific Networks. His expertise covers omics data integration and reconstruction of molecular networks, predictive modelling applying network and machine learning approaches. Main applications include the reconstruction of cancer-specific metabolic network models on genome scale. This allows to get a detailed understating of metabolic alterations in cancer and to identify sensitive intervention points for metabolism-based treatment of cancer. A similar methodology can be applied to other diseases with metabolic aspects.

discover CANBIO2
Projects – WP1 – Novel Cancer Models
Projects – WP2 – Cancer Metabolism
Projects – WP3 – Novel Treatment Strategies
Contact
For any question related to CANBIO2, please contact: